COVID-19's prolonged asymptomatic period presents a unique set of challenges. Accurate diagnosis, contact tracing, and isolation will remain the cornerstones of Federal, State, and Local response efforts well into the future.
PCR and related nucleic acid detection systems will remain the gold standards for this kind of testing, however, cost, throughput, and contact requirements make current systems unsuitable for the kind of population-scale diagnostic testing needed to control COVID-19 now, and as we begin to return to a more normal pace of life.
We have designed a protocol that enables fast, affordable, population-scale COVID-19 diagnostic testing using Next Generation Sequencing, and we are working toward deployment. To learn more or to help, please get in touch.
get in touch
As part of our response to COVID-19, we are making our core computational antibody optimization offering available to any organization attempting to produce anti-COVID-19 antibodies - completely free of charge.
We can work with data from both in vitro and from immunization campaigns (with limitations) to generate antibodies with potential fitness advantages over those already discovered, and can also help design optimized in vitro libraries for subsequent rounds of selection. We are at an early stage of development, but think we have much to offer.
Email us at covidoptimization@angstrom.bio for details.
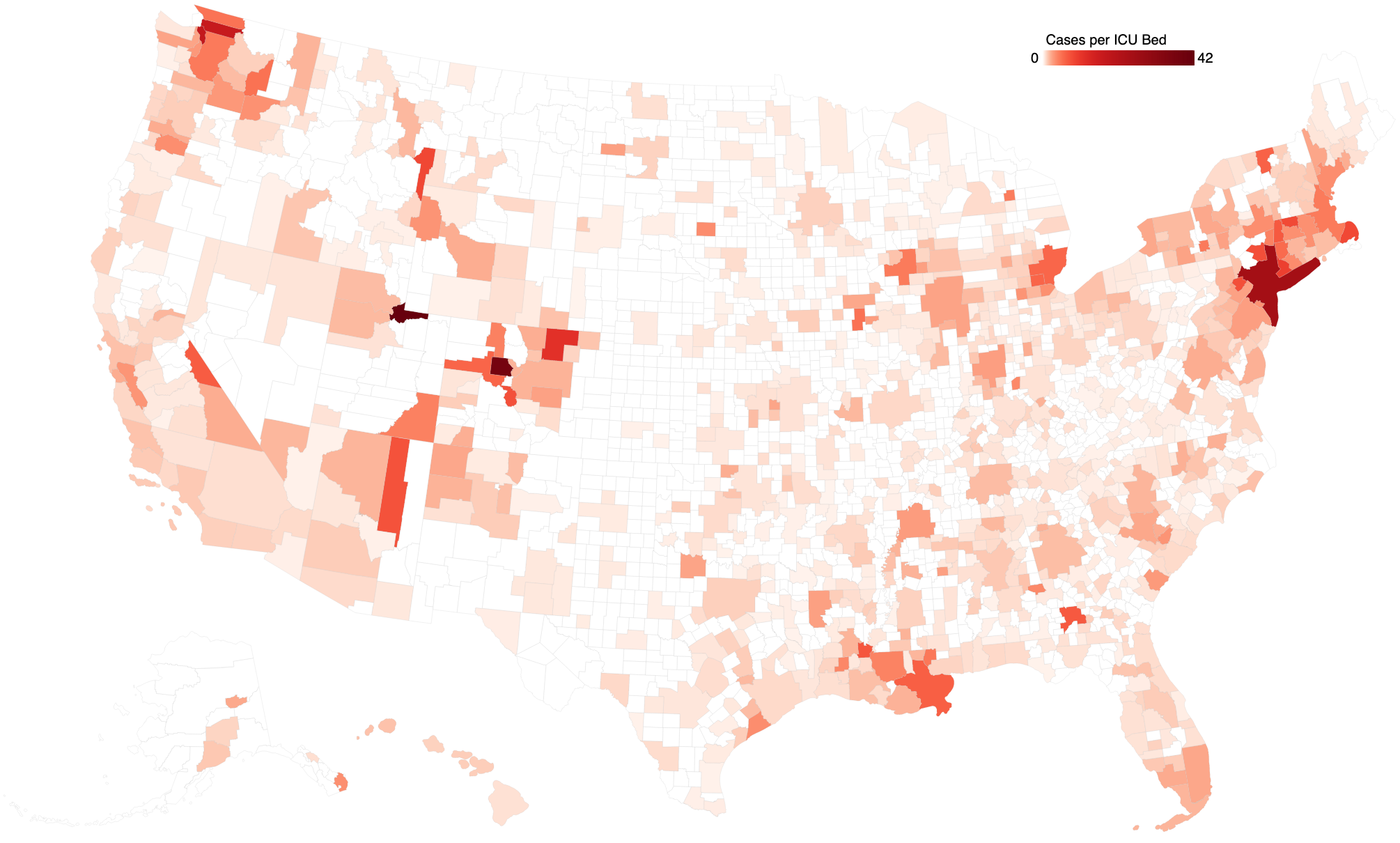
National and state-level counts of COVID-19 cases and of related data miss much of the story around COVID-19's actual impact.
We have produced an open resource for monitoring the risk to local and regional health care systems due to COVID-19, and for guiding determinations around response-related resource allocation.
We are using the map and related information tools to understand the material, equipment, personnel, and capital requirements for the launch of COVID-19 therapeutics and diagnostics.
Go to the Map